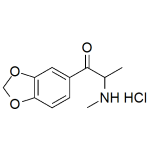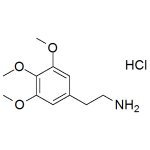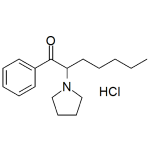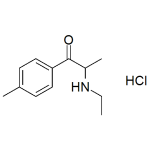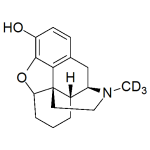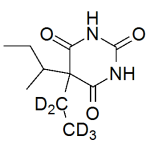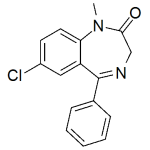Chemtos
Service, Quality, Speed
Chemtos
Service, Quality, Speed
Ref Stds
Methylone Hydrochloride
High purity Methylone Hydrochloride includes a comprehensive Certificate of Analysis and all supporting analytical data
Mescaline HCl
High purity Mescaline Hydrochloride (3,4,5-Trimethoxyphenethylamine, TMPEA, Hallucinex, Peyote, US DEA C-I 7381) includes a comprehensive Certificate of Analysis and all supporting analytical data
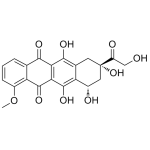
Doxorubicinone
High purity Doxorubicinone includes a comprehensive Certificate of Analysis and all supporting analytical data
Ketamine-d4 0.1mg/ml
High purity Ketamine labeled d4 Hydrochloride solution includes a comprehensive Certificate of Analysis and all supporting analytical data
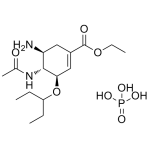
Oseltamivir Phosphate
High purity Oseltamivir Phosphate includes a comprehensive Certificate of Analysis and all supporting analytical data
Duloxetine-d7 HCl 0.1mg/ml
High purity Duloxetine labeled-d7 HCl solution includes a comprehensive Certificate of Analysis and all supporting analytical data
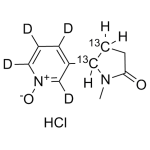
Cotinine-N-Oxide labeled 13C2,d4 Hydrochloride
High purity Cotinine-N-Oxide labeled 13C2,d4 Hydrochloride includes a comprehensive Certificate of Analysis and all supporting analytical data
PV8 HCl (alpha-PHPP)
High purity PV8 HCl (alpha-pyrrolidinoheptaphenone, α-pyrrolidino-heptaphenone, α-PHPP, α-PEP) includes a comprehensive Certificate of Analysis and all supporting analytical data
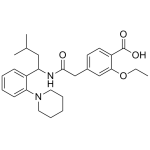
Repaglinide
High purity Repaglinide includes a comprehensive Certificate of Analysis and all supporting analytical data
4-MEC HCl (4-Methylethcathinone HCl)
High purity 4-MEC HCl (4-Methylethcathinone Hydrochloride) includes a comprehensive Certificate of Analysis and all supporting analytical data
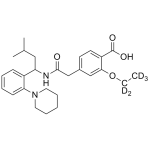
Repaglinide - Labeled d5
High purity Repaglinide - Labeled d5 includes a comprehensive Certificate of Analysis and all supporting analytical data
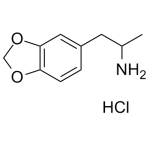
MDA HCl 1mg/ml
High purity MDA HCl (3,4-Methylenedioxyamphetamine) solution includes a comprehensive Certificate of Analysis and all supporting analytical data
Desomorphine-d3
High purity Desomorphine labeled d3 includes a comprehensive Certificate of Analysis and all supporting analytical data
Butabarbital labeled d5
High purity Butabarbital labeled d5 includes a comprehensive Certificate of Analysis and all supporting analytical data
Diazepam
High purity Diazepam includes a comprehensive Certificate of Analysis and all supporting analytical data